 |
| Hi |
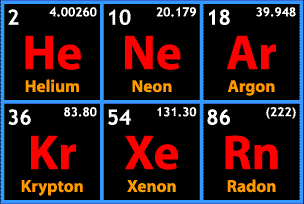
So! What is special about this group of elements? Are they explosive? Poisonous? Metalloid? No, no, and no. The distinguishing characteristic of the noble group is more what they do not do. As we've worked our way across the periodic table, I'm sure you've noticed that elements are almost never found as a group of unconnected atoms. They may bond in pairs of atoms to form a gas, like nitrogen, or perhaps engage in the complex bonding we see in the transition metals. Maybe they form covalent bonds, maybe they form ionic bonds. Perhaps they bond to other atoms of the same element, perhaps they join with atoms of other elements to form compounds, but just about everything out there tends to form bonds. Except for the noble gases.
 |
| Weirdos |
This is a concept that has come up repeatedly over the course of Chemistry Wednesdays, but here it is again; valence shells. Remember, all of the varieties of chemical bonds we've seen have involved that outermost theoretical shell of electrons. Atoms combine with other atoms in order to achieve a full outermost shell, or orbital. You may have noticed a trend as we have moved across the table. Way back on the left side, in the alkali metals, that outermost shell had only one electron. By the time we got to the halogens, that outermost shell was only one electron short of a full set. That trend is real, and here, at the right side of the table, we reach atoms that naturally have a full valence shell.
 |
| Atoms don't look like this in real life, but let's pretend. |
If you're thinking that's a little odd, you are not alone. In fact, the periodic table, as originally conceived by Mendeleev, did not allow for such a thing. However, in 1895, two British chemists discovered argon; while they initially wondered if it wasn't simply a variation of nitrogen, it quickly became clear that there was a whole new family of elements out there to be discovered. The ability of the noble gases to exist in an unbonded (or monatomic) state was one of the things that pointed early 20th century chemists towards a more accurate understanding of electron bonding.
As a group, then, the noble gases are incredibly unreactive. This was a contributing factor in why it took us so long to learn of their existence. Now, just because the group is unreactive does not mean it never reacts. No compounds have been made containing helium or neon, but argon, krypton, and xenon compounds have been created, and have a few chemical and industrial uses. For the most part, though, we're most likely to encounter them in their monatomic, gaseous forms.
And encounter them we do! Let's start at the top, with helium. Helium is the second-most abundant element in the universe (after hydrogen); it is a major component of the Sun, and is present in trace quantities in Earth's atmosphere. Pure helium gas has a density about one-seventh that of air on Earth, and is used in lighter-than-air craft, from hot air balloons to party balloons. Helium has so many applications (everything from MRIs to silicon computer chip manufacture) that we're actually facing a helium shortage. Well, high demand, limited availability on Earth, and some odd quirks of government policy.
 |
| Brought to you by helium. And a tremendous yardage of nylon. |
Neon might be most familiar from the eponymous lights. Actually, all the noble gases can be used to create the so-called gas discharge decorative lighting, which is commonly called neon lighting. In this form of lighting, a noble gas is exposed to electrical energy. The energy "excites" the electrons; they briefly jump to a theoretical higher energy level, then return to their original energy level, and emit a colorful glow in the process. Neon is also used as a coolant, and in the manufacture of lasers.
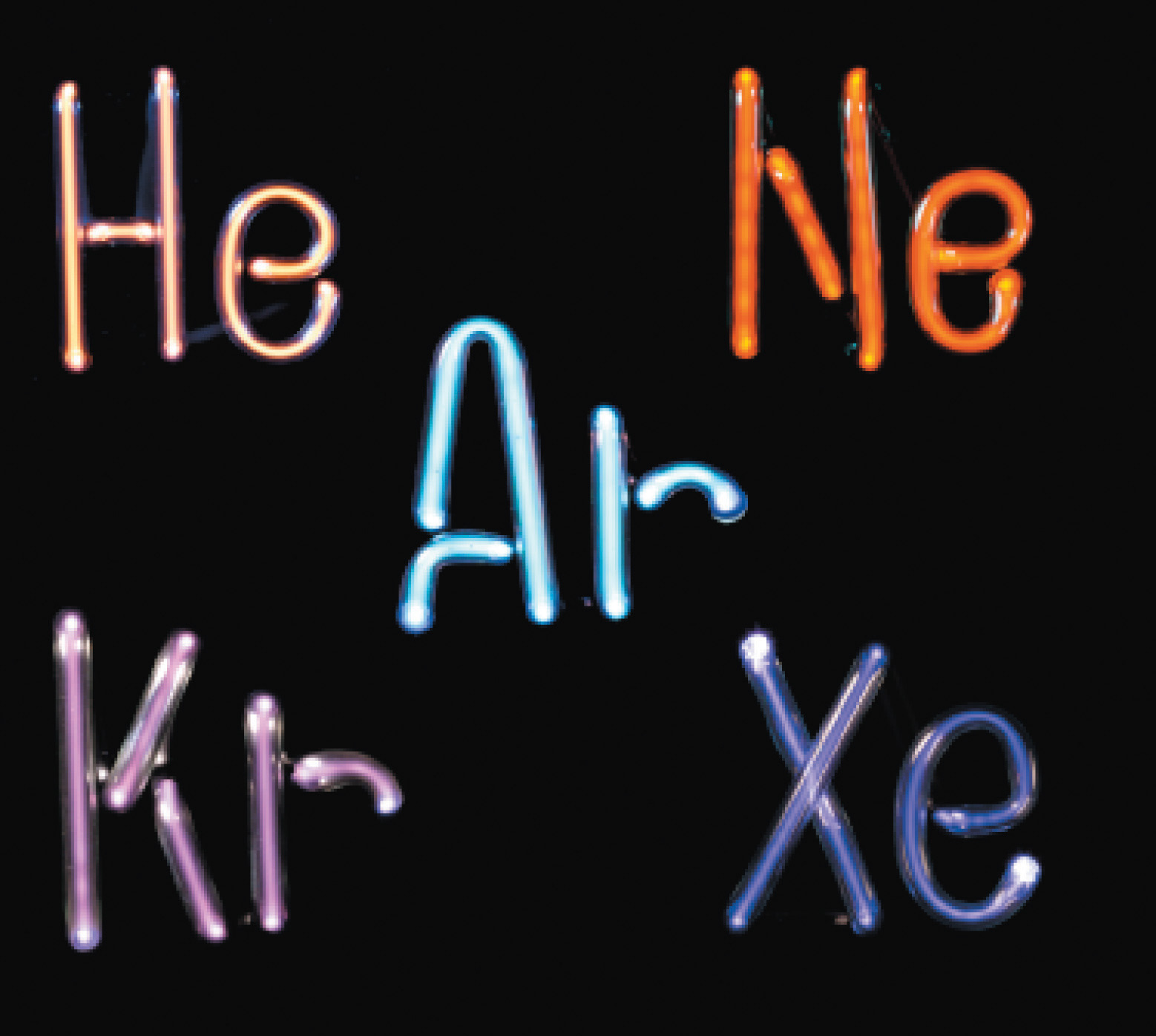 |
| And yes, these are the appropriate colors for each element. |
Argon is the third most abundant gas in Earth's atmosphere (after nitrogen and oxygen). There are a number of industrial uses for argon, mostly stemming from its lack of reactivity; argon gas is used to buffer reactions and equipment that might be damaged by interaction with other, more reactive gases (I'm looking at you, oxygen).
 |
| Other uses include lovely blue and violet lights. |
Krypton, in addition to being Superman's home planet, has a few uses in flash photography, and is used in some fluorescent light bulbs. Krypton is difficult to obtain, which has limited its industrial uses.
 |
| Interestingly enough, krypton, unlike Kryptonite, is not a sickly greenish color. |
Xenon turns up in strobe lighting, and car headlights. Certain kinds of xenon lamps emit a light strong enough to kill bacteria, and xenon has been used as a component of experimental rocket fuel.
| Xenon in action. |
Radon is the only radioactive noble gas. It forms as a component of uranium decay; since uranium is present throughout the Earth's crust, radon can and does form anywhere. Depending on the underlying soil and rocks of a given area, radon can accumulate in homes, where the radioactive gas is inhaled, to the detriment of the one inhaling. Radon is the leading cause of lung cancer amongst non-smokers. It is recommended that people living in areas prone to radon accumulation regularly test their homes and workplaces for the gas. The month of January is National Radon Action Month in the United States, and even if you don't live in the US, it's good to make sure that radon is not an issue in any buildings where you might spend a lot of time!
 |
| It's serious stuff |
There's the noble gases for you. From hot air balloons to radioactive gas, they get a lot done for a group that was once called the "inert gases". Next week, we'll finish out the table with a look at those weird "uup" and "uus" elements you see hanging out in the bottom right row. It's been a long ride; thanks for sticking around!
No comments:
Post a Comment